Our story
THE DCCC ctDNA RESEARCH CENTER
In January 2020 the Danish Cancer Society established The Danish National Center for Circulating Tumor DNA Guided Cancer Treatment in collaboration with DCCC - Danish Comprehensive Cancer Center. The ctDNA Research Center is supported by funds from 'Knæk Cancer' and works to strengthen the Danish national collaboration in ctDNA cancer research - for the benefit of every Dansih cancer patients.
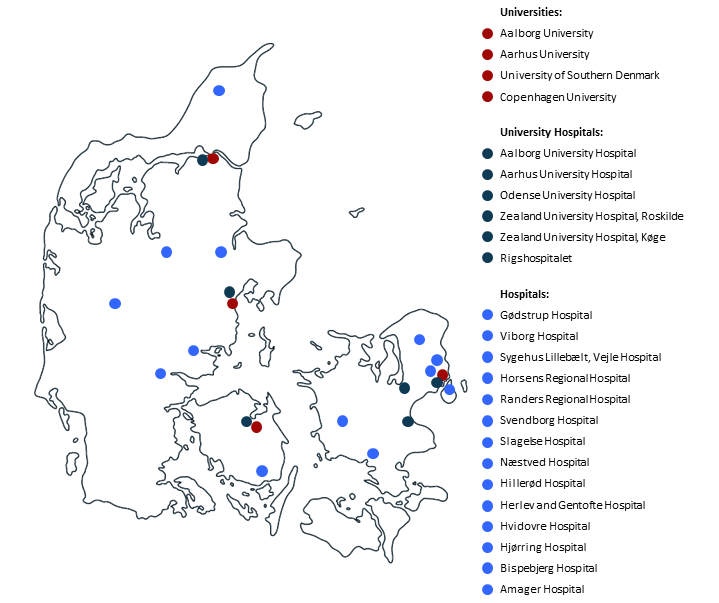
More than 60 medical doctors and researchers from 4 universities and 17 hospitals, spanning all five regions of the country, was involved in the establishment of the ctDNA Center.
Background
The Center
In January 2020 the Danish National Center for Circulating Tumor DNA Guided Cancer Treatment was established as a 'brickless' center for a 5-year funding period. The objective of the center is to provide an effective and vital framework for optimal implementation of evidence-based ctDNA usage in cancer patient management in Denmark.
With the center all Danish clinicians and researchers with an interest in using ctDNA based analysis for monitoring and decision making are brought together to facilitate ctDNA based research and clinical implementation of ctDNA guided patient stratification.
The overall aim is to support national research initiatives in early detection and personalized cancer treatment. The center includes research projects focusing on a wide range of cancer diseases, covering screening, treatment, and early detection of relapse.
Purpose
National collaboration
The purpose of the ctDNA Center is to establish a unique national research network. With the center all Danish clinicians and researchers with an interest in using ctDNA based analysis for monitoring and decision making are brought together to facilitate ctDNA based research and clinical implementation of ctDNA guided patient stratification. The center promotes and facilitates ctDNA research, clinical trails, and translation into clinical practice.
The objective of the center is to provide an effective and vital framework for optimal implementation of evidence-based ctDNA usage in cancer patient management in Denmark. We work to make the clinical benefits of ctDNA guided clinical decision-making available to all Danish cancer patients regardless of where in the country they live and no matter what type of cancer they have.
Our values
Essense of the center
National collaboration
At the heart of the center is its mission to unite Danish clinicians and researchers who share an interest in ctDNA.
Individual treatment
We want to be able to give the right treatment at the right moment to the right person.
Knowledge sharing
The center provides a platform for knowledge sharing across Denmark, scientific background0, and cancer types.
Faster implementation
We believe that national collaboration and shared infrastructures will make the clinical implementation easier.
Achieving synergy
A collective and coordinated effort to optimize results through the effective interaction of all involved researchers.
Focus
The center focus on ctDNA guided cancer treatment
Biomarkers for personalized risk-stratification are urgently needed. Consequently, clinical practice is dominated by “one size fits all” strategies e.g. for treatment-and post-treatment surveillance. This often leads to massive overtreatment and a waste of resources following up non-relapsing patients.
The center aims to offer participation in evidence generating clinical trials to all relevant Danish cancer patients. Such trials will explore the clinical utility of using ctDNA for e.g. detection of cancer in asymptomatic individuals, post-operative detection of residual disease to guide adjuvant treatment decisions, detection of residual disease after adjuvant therapy in order to guide imaging surveillance intensity, for blood-based recurrence surveillance, for monitoring therapy response (neoadjuvant, adjuvant and in treatment of metastatic disease), detection of actionable targets for guiding therapy choice etc.
If the results confirm our hypotheses: that ctDNA guided management has clinical benefit compared to the present standard, and that ctDNA guided management is cost-effective, then we shall provide business analyses and other decision and implementation supporting information to the hospitals and health regions. We anticipate timely and relatively straight forward implementation of ctDNA guided cancer management at a national scale in Denmark, since the conducted studies will be national with participation from the majority of the clinical centers treating cancer patients in Denmark.
With the center all Danish clinicians and researchers with an interest in using ctDNA based analysis for monitoring and decision making are brought together to facilitate ctDNA based research and clinical implementation of ctDNA guided patient stratification.
National collaboration
Involved departments
As indicated on the map institutions (universities and hospitals) from all over Denmark are involved in and a member of the DCCC ctDNA Research Center. We have participation from all the departments involved in management of cancer patients e.g. Surgery, Pathology, Oncology, Radiology, Nuclear medicine, Genomic medicine, and Molecular medicine. Thus, making it a multidisciplinary research center.
Our organization
It takes all of us to make a national collaboration
CENTER PARTICIPANTS
Our members are from different universities and hospitals across Denmark - working with research in ctDNA guided cancer treatment. Participants represents diverse departments integral to the management of cancer patients. including Surgery, Pathology, Oncology, Radiology, Nuclear medicine, Genomic medicine, and Molecular Medicine. This reflects the multidisciplinary nature of our research center.
STEERING COMMITTEE
The center operates under a Steering Committee, featuring a representative from each participating department. This committee ensures a broad scientific and clinical anchoring of the center in the local department and institution. Additionally, the Steering Committee is responsible for making decisions on behalf of the center.
EXECUTIVE COMMITTEE
Among the members of the Steering Committee, an Executing Committee has been apointed. The Executing Committee prepare matters for consideration in the Steering Committee. There is a geographical and scientific broadness in the committee.
CENTER MANAGEMENT
Professor Claus Lindbjerg Andersen is Center Director and Professor Lars Dyrskjøt Andersen is Center Vice Director. Together with Scientific Coordinator Anne Lærke Lorentzen the center management is responsible for the daily management and facilitation of the tasks in the center.
Contact
Find contact information here
Center membership
Learn what a center affiliation means
ADDRESS FOR THE SECRETARIAT
Science Center Skejby, MOMA
Brendstrupgårdsvej 21, build. A
8200 Aarhus N
CONTACT
ctDNA@clin.au.dk
+45 78 45 53 39